
2025-10-17 Posted by TideChem view:400
In recent years (2018-2024), the U.S. Food and Drug Administration (FDA) approved an average of approximately 50 new drugs annually. Notably, biologics have accounted for a significant proportion of these approvals, with 14, 15, 17, and 16 biologics approved in 2021, 2022, 2023, and 2024 respectively, representing about one-third of total annual approvals.
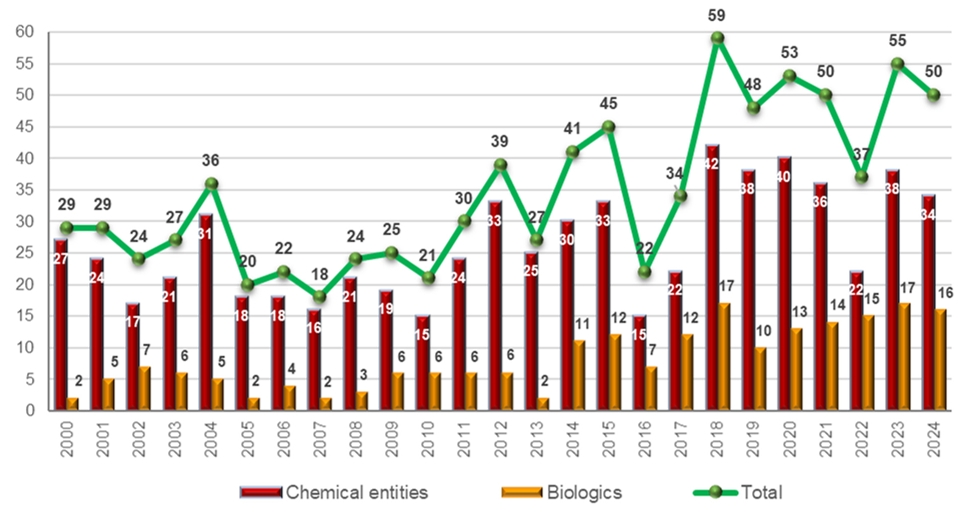
Figure 1. Drugs (new chemical entities and biologics) approved by the FDA in the last 25 years.
Long-acting technologies for biologics, especially half-life extension strategies for protein and peptide therapeutics, have emerged as a major research focus in the pharmaceutical industry. Regulatory data show that the FDA has approved 11 PEGylated drugs in the past four years, fully demonstrating their excellent physicochemical properties and pharmacokinetic profiles. To understand the different types of PEG linkers and their applications, see our comprehensive guide What are PEG Linkers?
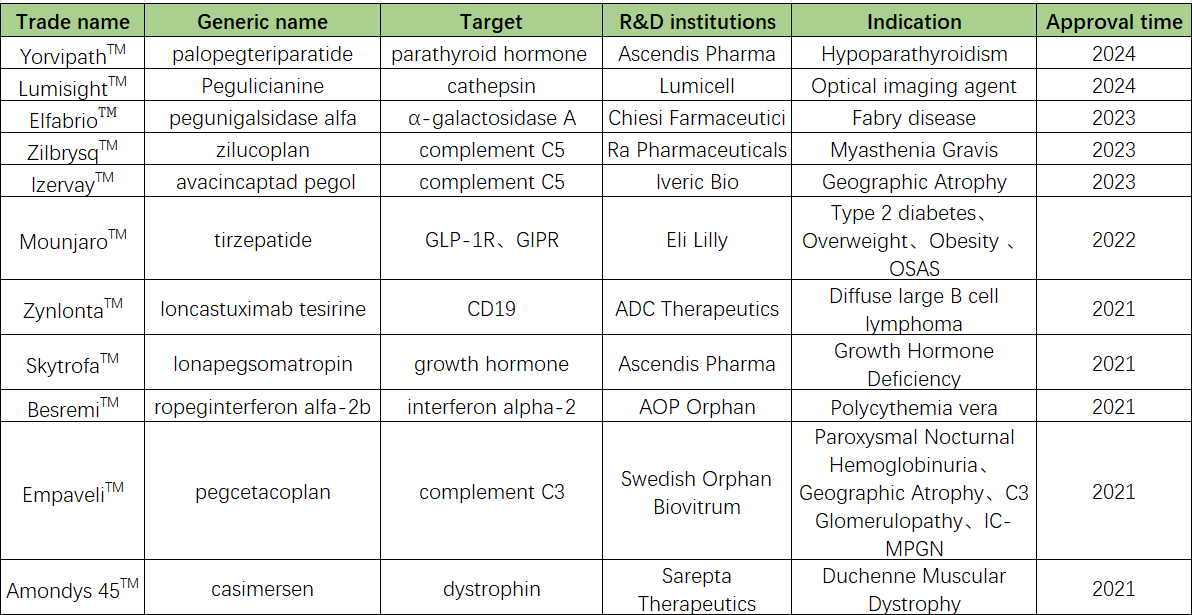
Figure 2. PEGylated Drugs Approved by the FDA in the Past Four Years.
PEGylation is a popular method in drug development these days. It's used a lot in FDA-approved medicines because it has some important benefits:
Better Solubility and Stability
PEG chains make biomolecules dissolve in water better, which stops them from clumping together and forming solids. This helps the drug stay stable and last longer on the shelf, which is important for getting FDA approval.
Longer Circulation and Less Clearing
PEGylation hides drugs so they don't get cleared out of the body too quickly by the kidneys or broken down by enzymes. This means the drug lasts longer, so patients don't have to take it as often, which makes it work better.
Less Likely to Cause Immune Reactions
Drugs with PEG attached are less likely to cause immune reactions compared to drugs without it. The FDA pays close attention to this during their review.
Works with Different Types of Molecules
PEGylation can be used with proteins, peptides, enzymes, oligonucleotides, and antibody-drug conjugates (ADCs). This makes it a useful tool for treating all kinds of diseases, from rare ones to cancer.
| Drug Name | Year Approved | PEG Type / Linker | PEG Product Category | Indication | Molecule Type |
| Palopegteriparatide (Yorvipath™) | 2024 | Branched mPEG via TransCon | Branched PEG | Hypoparathyroidism | Peptide hormone |
| Pegulicianine (Lumisight™) | 2024 | Polydisperse mPEG | m-PEG | Cancer imaging | Peptide‑fluorophore conjugate |
| Pegunigalsidase alfa (Elfabrio™) | 2023 | Short PEG moieties | m-PEG | Fabry disease | PEGylated enzyme |
| Zilucoplan (Zilbrysq™) | 2023 | Large PEG side chain | m-PEG | gMG | Peptide inhibitor |
| Avacincaptad pegol (Izervay™) | 2023 | Dual PEG chains | Branched PEG | Geographic atrophy | Aptamer pegylation |
| Tirzepatide (Mounjaro™) | 2022 | Mini‑PEG moieties | m-PEG | T2D, obesity | Dual incretin agonist |
| Ioncastuximab tesirine (Zynlonta™) | 2021 | PEG linker + PABC | Maleimide Linkers | DLBCL | ADC (CD19 targeting) |
| lonapegsomatropin (Skytrofa™) | 2021 | PEG via TransCon | m-PEG | Growth hormone deficiency | PEG‑prodrug |
| Ropeginterferon alfa‑2b (Besremi™) | 2021 | PEG conjugated | m-PEG | Polycythemia vera | PEGylated interferon |
| Pegcetacoplan (Empaveli™) | 2021 | PEG via AEEA linker | m-PEG | PNH | Complement C3 inhibitor |
| Casimersen (Amondys 45™) | 2021 | Mini‑PEG | m-PEG | Duchenne muscular dystrophy | Antisense oligonucleotide |
Palopegteriparatide is a hormone replacement therapy approved by the FDA in 2024 for the treatment of hypoparathyroidism. It consists fragment 1–34 of the human parathyroid hormone (PTH), where the terminal Aib residue is conjugated to a branched methoxypolyethyleneglycol (mPEG) carrier via a TransCon linker.
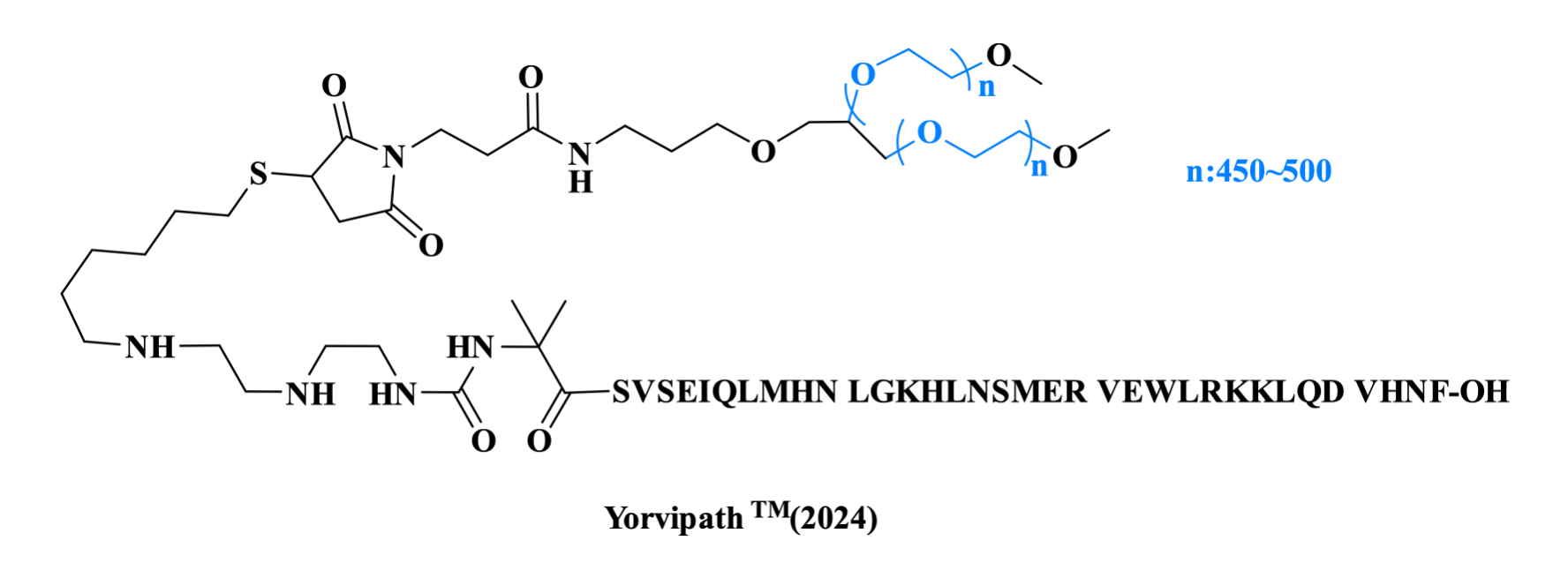
Figure 3. The structure of YorvipathTM.
Another PEGylated drug approved by the FDA in 2024 is Pegulicianine, an optical imaging agent for the detection of cancerous tissues. The molecular architecture utilizes a linear peptide scaffold (Ahx-Gly-Gly-Arg-Lys-AEEA-Cys-NH₂) , where the amino of the Ahx is linked to a dark quencher (QSY21), the ε- NH₂ of the Lys to the fluorophore (Cy5), and the thiol of Cys to a polydisperse mPEG, which enhances drug solubility.
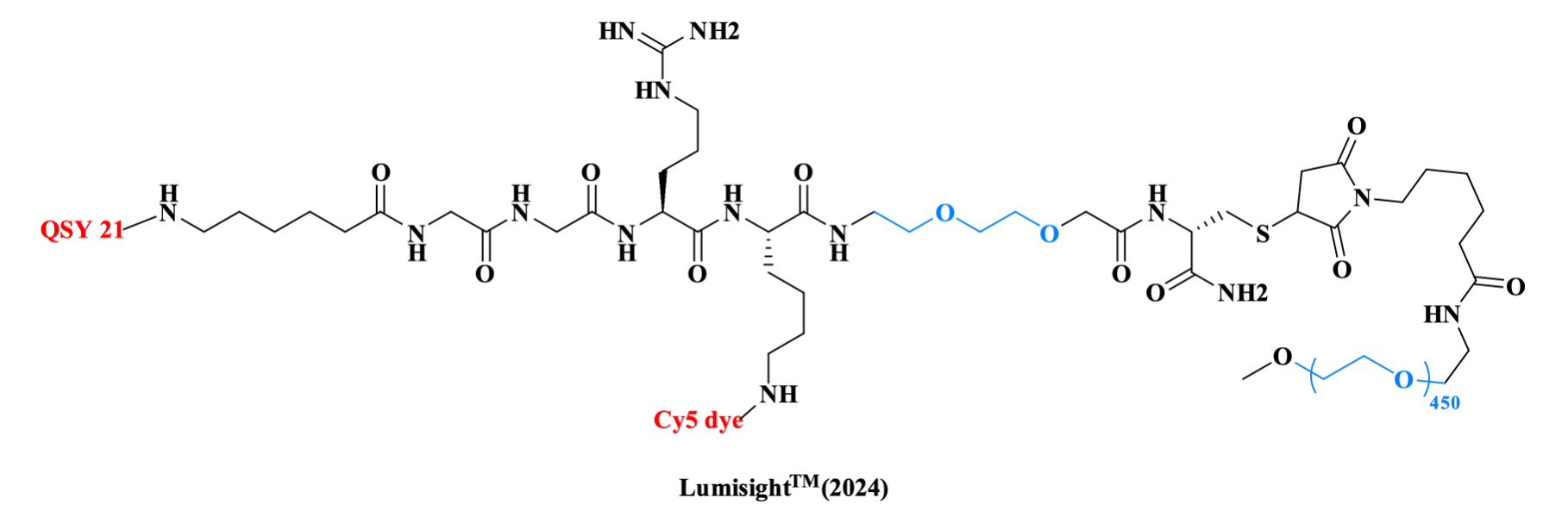
Figure 4. The structure of LumisightTM.
Pegunigalsidase alfa, a PEGylated enzyme replacement therapy (ERT) to treat Fabry disease, is a plant cell culture-expressed, and chemically modified stabilized recombinant version of the α–Galactosidase–A enzyme. Protein sub-units are covalently bound via chemical cross-linking using short PEG moieties, resulting in a molecule with stable pharmacokinetic parameters. In clinical studies, Pegunigalsidase alfa has been observed to have an initial half-life of 78.9 ± 10.3 hours.
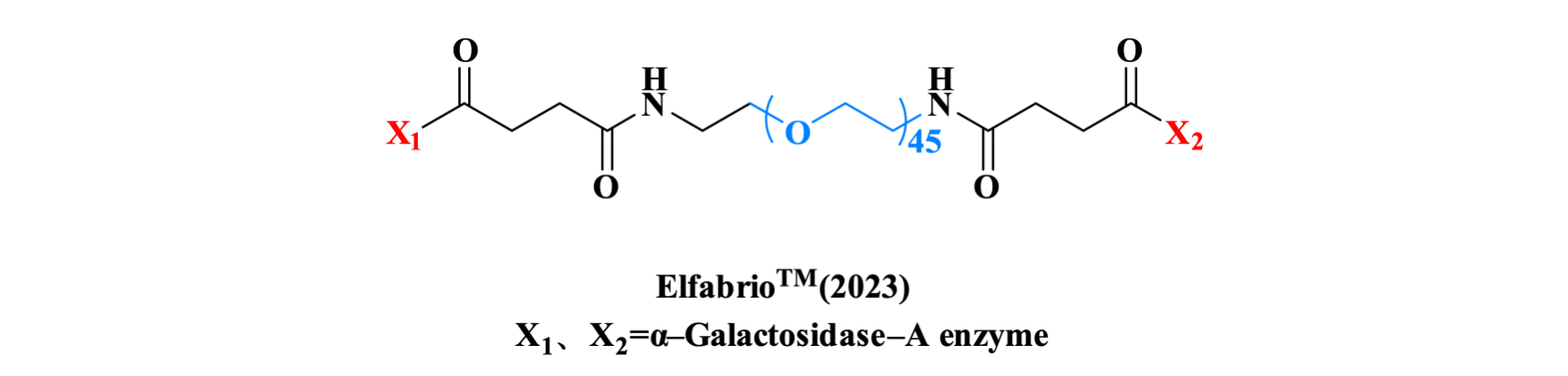
Figure 5. The structure of ElfabrioTM.
Zilucoplan is a once-daily SC, self-administered peptide inhibitor of complement component 5 (C5 inhibitor). In October 2023, zilucoplan was approved by the U.S. Food and Drug Administration (FDA) for the treatment of gMG in adult patients who are anti-acetylcholine receptor (AchR) antibody positive. Its backbone consists of 15 amino acids. The ε-amino group of the Lys1 and the β-carboxyl group of the Asp6 are covalently linked via an amide bond. A Lys residue situated at the C-terminal part bears a side chain containing a large PEG and a fatty structure (palmitic acid) linked by a γ-Glu residue.
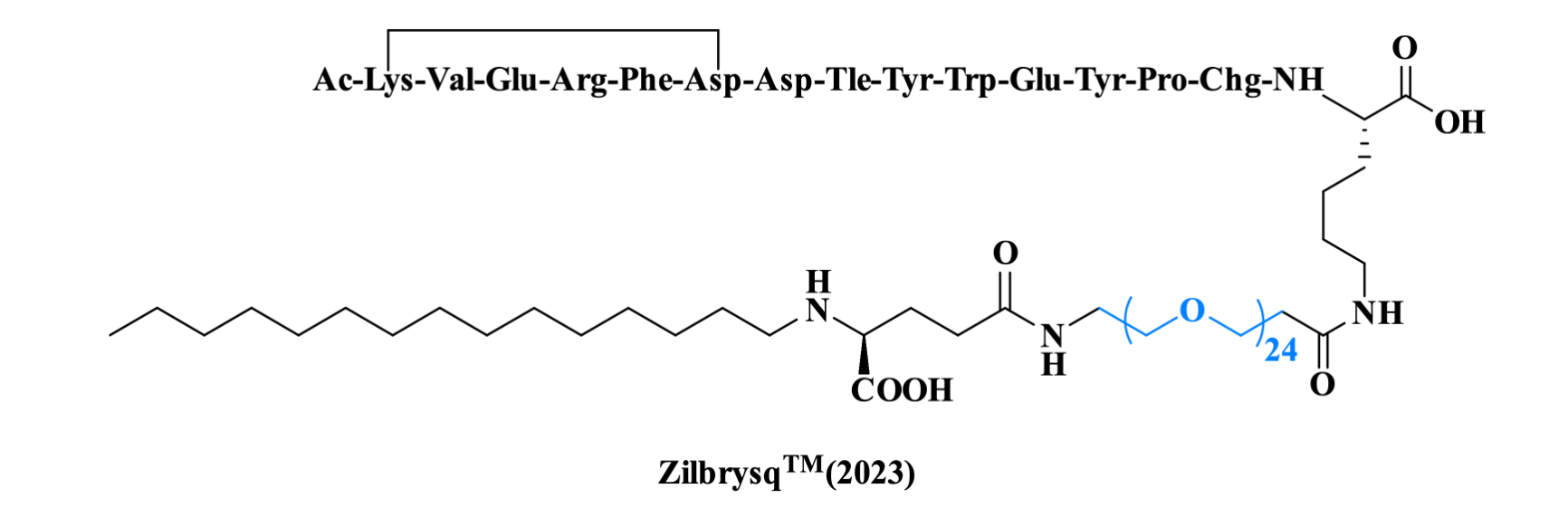
Figure 6. The structure of ZilbrysqTM.
Avacincaptad pegol, developed by Iveric Bio, is a C5 inhibitor that has been shown to slow geographic atrophy progression by targeting the source of retinal cell death while preserving the upstream benefits of the complement system. The aptamer Contains 39 nucleotides, with two PEG chains conjugated at the 5' end to extend the plasma half-life of the drug in vivo.
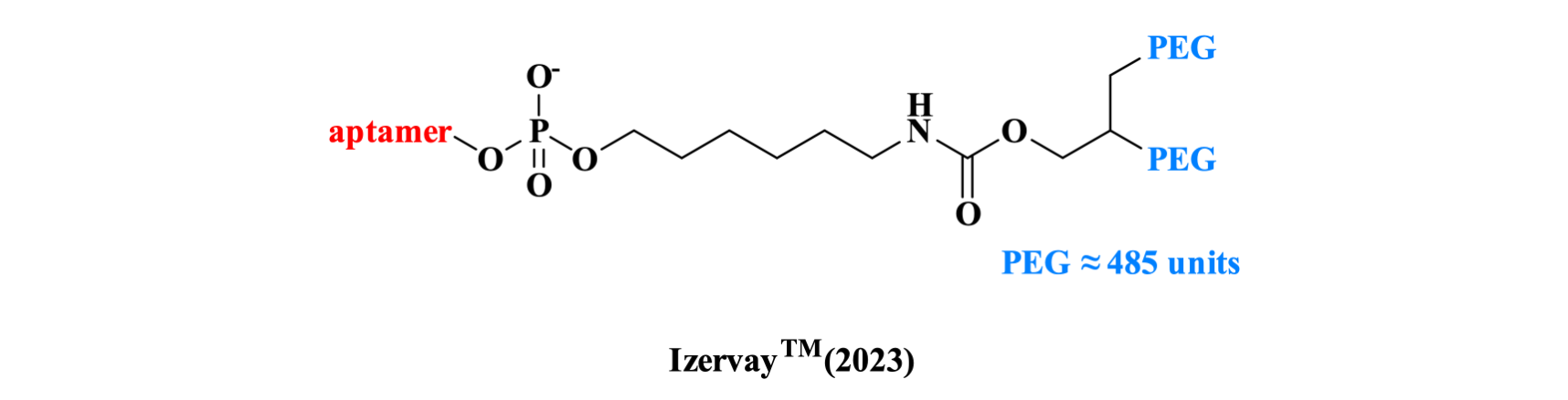
Figure 7. The structure of ZilbrysqTM.
Tirzepatide, the only PEGylated drug approved by the FDA in 2022, is indicated for type 2 diabetes (T2D), obesity, overweight, and obstructive sleep apnea syndrome (OSAS). It is a dual GIP and GLP-1 receptor agonist composed of 39 amino acids. The sidechain is formed by C20 diacid linked through the γ-glutamic acid and two two mini-polyethyleneglycol moieties (AEEA) to the ε-amino of the Lys20.
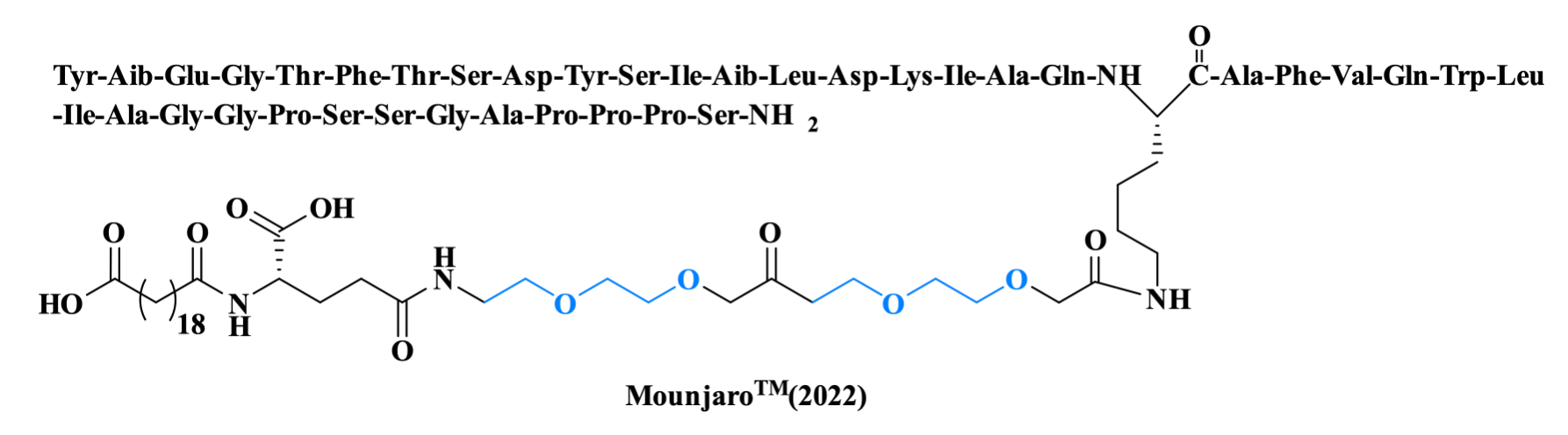
Figure 8. The structure of MounjaroTM.
The CD19-targeting ADC drug loncastuximab tesirine was approved by the FDA in 2021 for the treatment of diffuse large B cell lymphoma (DLBCL). In this drug, the mAb is conjugated via a Cys to a maleimide-based moiety, which is further linked through a PEG chain to the Val-Ala dipeptide and to the PABC. The payload SG3199 is then attached to the PABC.
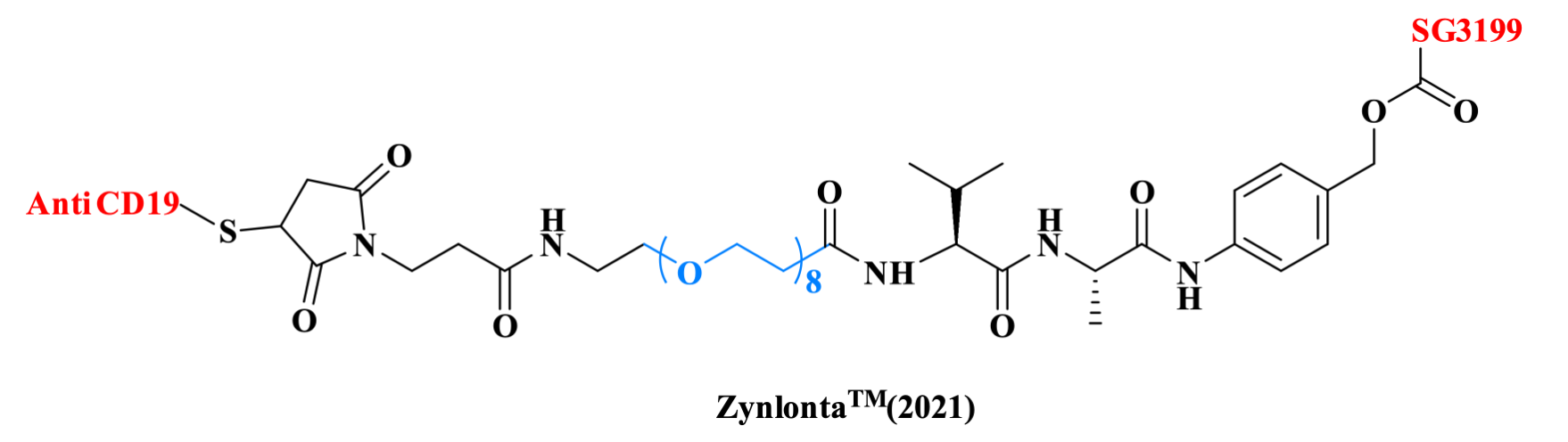
Figure 9. The structure of ZynlontaTM.
Ionapegsomatropin is the first FDA-approved weekly growth hormone injection for treating growth hormone deficiency (GHD) in patients aged ≥1 year and weighing ≥11.5 kg. As the first long-acting growth hormone prodrug utilizing TransCon technology, it is formed by linking human growth hormone (hGH) to a PEG china via a TransCon linker.
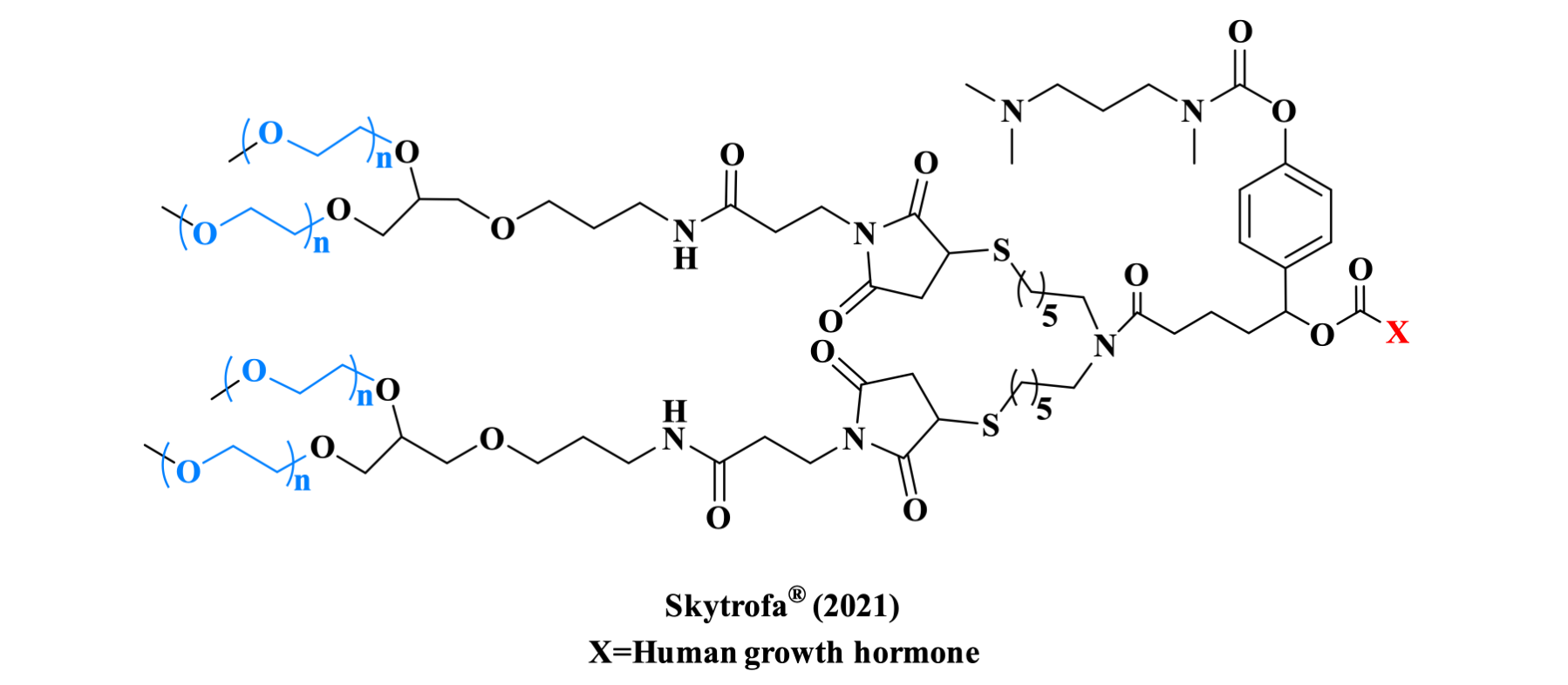
Figure 10. The structure of Skytrofa TM.
Ropeginterferon alfa-2b is the first FDA-approved medication for polycythemia vera that patients can take regardless of their treatment history, and the first interferon therapy specifically approved for polycythemia vera. Ropeginterferon alfa-2b is formed by covalent conjugation of a PEG moiety to the Pro N-terminal residue of recombinant IFNα2 analogues, allowing for subcutaneous administration once every two weeks.
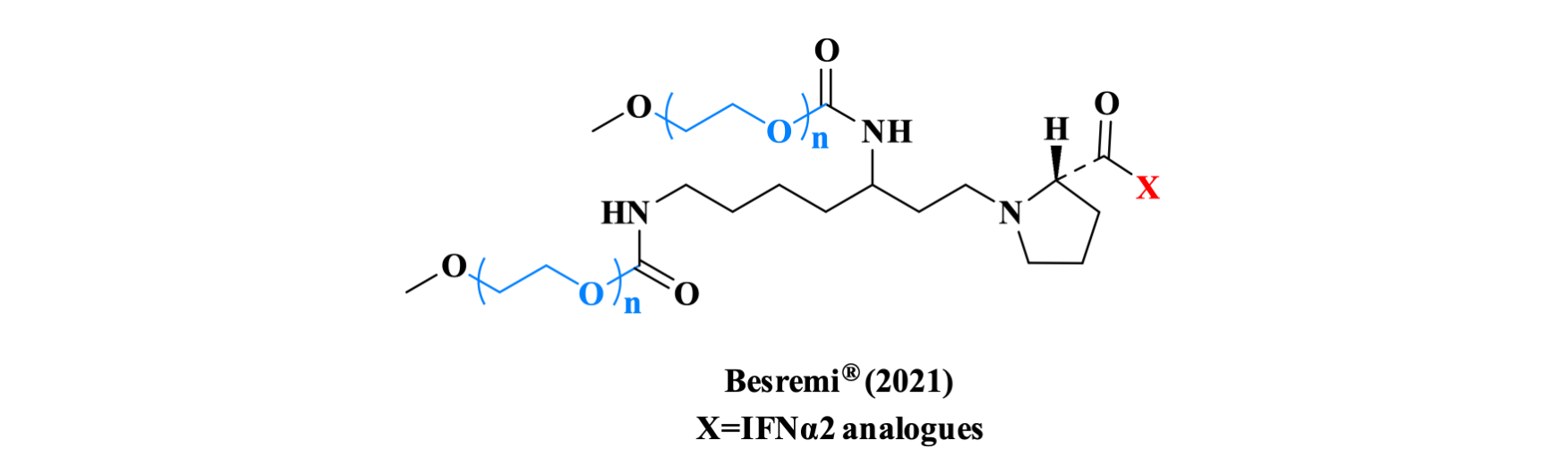
Figure 11. The structure of Besremi TM.
Pegcetacoplan targets complement C3 protein and is indicated for treating paroxysmal nocturnal hemoglobinuria (PNH) and other complement-mediated disorders, having received FDA accelerated approval in 2021. The peptide structure features an intramolecular disulfide bond between two cysteine side chains, while the terminal Thr is conjugated to a PEG chain via an AEEA spacer and Lys linker, extending plasma half-life to enable biweekly administration.
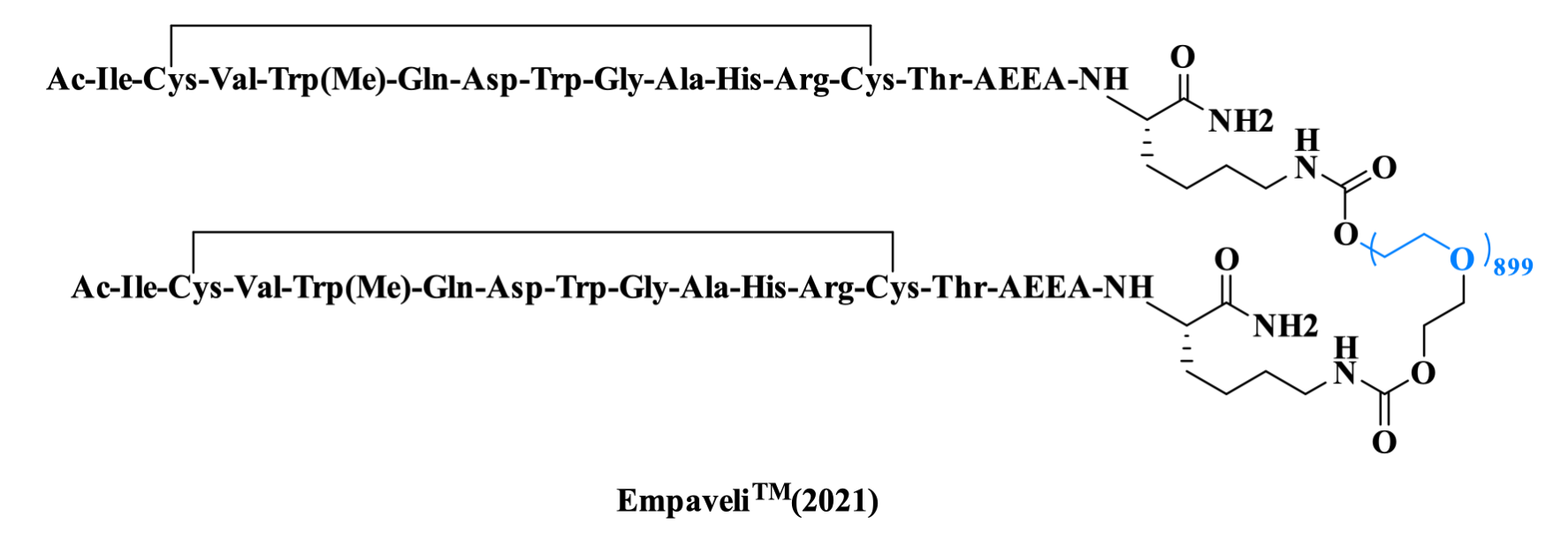
Figure 12. The structure of Empaveli TM.
Casimersen is an antisense oligonucleotide indicated for the treatment of Duchenne muscular dystrophy in patients who have a confirmed mutation of the DMD gene that is amenable to exon 45 skipping. The sequence of 22 mer contains 5 units of 5-methyluracil and the 5′ ends with a mini-PEG of 3 units.
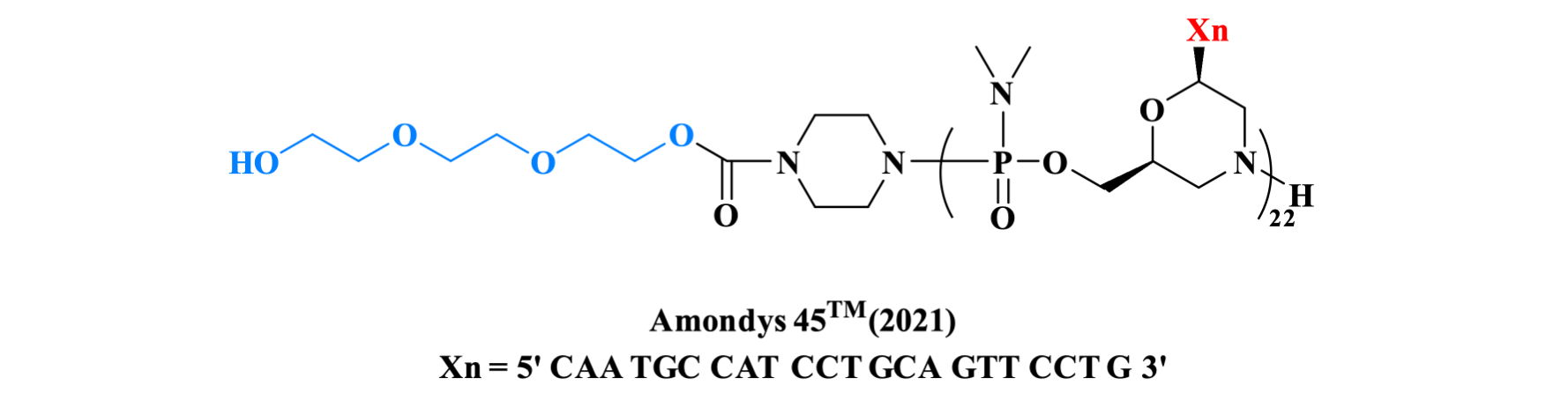
Figure 13. The structure of Amondys 45 TM.
Q1: What's PEGylation?
A1: PEGylation is when you attach polyethylene glycol (PEG) chains to biomolecules to help them dissolve, stay stable, and last longer in the body.
Q2: Why are FDA-approved drugs PEGylated?
A2: PEGylation helps drugs stay stable, last longer, cause fewer immune reactions, and make it easier for patients to stick to their treatment.
Q3: What kinds of PEG linkers are used?
A3: Some common ones are mPEG, Branched PEG, and Maleimide Linkers for ADCs.
Q4: Can PEGylation change how well a drug works?
A4: When designed well, PEG linkers can make a drug work better without getting in the way of it binding to its target. Though, if the PEG chains are too big, they can make the drug less active.
Q5: Are drugs with PEG safe?
A5: Drugs with PEG that get FDA approval are tested to make sure they're safe. PEGylation usually makes them less likely to cause immune reactions and side effects.
Understanding FDA-approved PEGylated drugs helps in designing effective bioconjugates. Learn more about PEG linker fundamentals and applications in What are PEG Linkers?
de la Torre, Beatriz G, and Fernando Albericio. “The Pharmaceutical Industry in 2024: An Analysis of the FDA Drug Approvals from the Perspective of Molecules.” Molecules (Basel, Switzerland) vol. 30,3 482. 22 Jan. 2025, doi:10.3390/molecules30030482.
de la Torre, Beatriz G, and Fernando Albericio. “The Pharmaceutical Industry in 2023: An Analysis of FDA Drug Approvals from the Perspective of Molecules.” Molecules (Basel, Switzerland) vol. 29,3 585. 25 Jan. 2024, doi:10.3390/molecules29030585.
de la Torre, Beatriz G, and Fernando Albericio. “The Pharmaceutical Industry in 2022: An Analysis of FDA Drug Approvals from the Perspective of Molecules.” Molecules (Basel, Switzerland) vol. 28,3 1038. 20 Jan. 2023, doi:10.3390/molecules28031038.
de la Torre, Beatriz G, and Fernando Albericio. “The Pharmaceutical Industry in 2021. An Analysis of FDA Drug Approvals from the Perspective of Molecules.” Molecules (Basel, Switzerland) vol. 27,3 1075. 5 Feb. 2022, doi:10.3390/molecules27031075.