
2025-11-28 Posted by TideChem view:256
Click chemistry is a highly efficient, precise, and rapid molecular construction technology. Its core principle is the flexible and controllable assembly of molecular modules using fast and highly selective chemical reactions. First proposed by Nobel Laureate Barry Sharpless in 2001, this concept exhibits the following outstanding characteristics:
(1)High Efficiency: The reactions proceed under mild conditions at a rapid rate, typically achieving yields exceeding 90% with minimal byproducts. The resulting products are easy to isolate and generally do not require chromatographic purification.
(2)Modularity: Utilizes standardized reactive groups, such as DBCO and azide, to enable the flexible coupling of different functional molecular modules.
(3)Bioorthogonality: The reactions can proceed specifically and safely even within living environments (e.g., cells, bloodstream), with almost no interference to normal physiological processes.
PEG linkers are a class of functional molecules known for their excellent hydrophilicity and biocompatibility. PEGylation can significantly enhance the water solubility and stability of drugs, thereby improving their physicochemical properties. Furthermore, the PEG chain acts as a flexible spacer, granting the connected functional modules an appropriate degree of motional freedom. Additionally, within the systemic circulation, PEGylation can delay drug clearance, effectively extending its half-life.
Depending on the specific click reaction type, particular chemical functional groups can be introduced at the terminal ends or side chains of PEG linkers to suit different conjugation needs. These linkers combine the excellent physicochemical properties of PEG with the highly efficient connection capability of click chemistry, and have been widely adopted in cutting-edge fields such as biopharmaceuticals, nanotechnology, and materials science.
Click chemistry gets a real boost in bioconjugation when you add PEG linkers that have special functions. By putting a PEG spacer between biomolecules and the stuff you want to attach, these linkers help things dissolve better in water, prevent crowding, and keep things spaced out just right during the attachment process. This means the reactions work better and you get more consistent results, especially when dealing with complicated biological stuff.
Besides speeding things up and making reactions more precise, PEG linkers also play a role in how stable the final product is, how it moves around the body, and how well it works overall. So, it's important to know how PEG linkers are built and what they do when you're planning bioconjugates that use click chemistry.For a comprehensive overview of PEG linker fundamentals, structures, and pharmaceutical applications, see What Are PEG Linkers?.
The choice of click reaction determines which PEG linker functional groups are most suitable for bioconjugation.
| Click Reaction Type | Typical PEG Functional Groups | Key Features | Common Bioconjugation Applications |
| CuAAC (Copper-Catalyzed Azide–Alkyne Cycloaddition) | Azide PEG, Alkyne PEG | High reaction efficiency and robustness; requires Cu(I) catalyst, which may cause cytotoxicity | Small-molecule conjugation, materials science, in vitro labeling |
| SPAAC (Strain-Promoted Azide–Alkyne Cycloaddition) | DBCO PEG, BCN PEG, Azide PEG | Copper-free, bioorthogonal, compatible with living systems; good balance of speed and stability | Protein and peptide conjugation, ADC development, in vivo labeling |
| IEDDA (Inverse Electron-Demand Diels–Alder Reaction) | Tetrazine PEG, TCO PEG | Extremely fast kinetics; catalyst-free; high selectivity under mild conditions | Live-cell imaging, rapid bioconjugation, advanced diagnostic probes |
Table: Common Click Chemistry Reactions Used with PEG Linkers
DBCO PEG integrates a dibenzocyclooctyne (DBCO) group with a polyethylene glycol (PEG) chain, making it an ideal choice for applications such as biomarking, bioconjugation, and surface modification. DBCO is a highly reactive cycloalkyne compound that undergoes a strain-promoted azide-alkyne cycloaddition (SPAAC) with azides, forming a stable triazole ring. This reaction is catalyst-free, exhibits high reaction rate and selectivity, and can proceed in either organic solvents or aqueous buffers, depending on the solubility and properties of the substrate molecules. The PEG chain enhances the water solubility and biocompatibility of therapeutic agents, thereby prolonging their systemic circulation time.
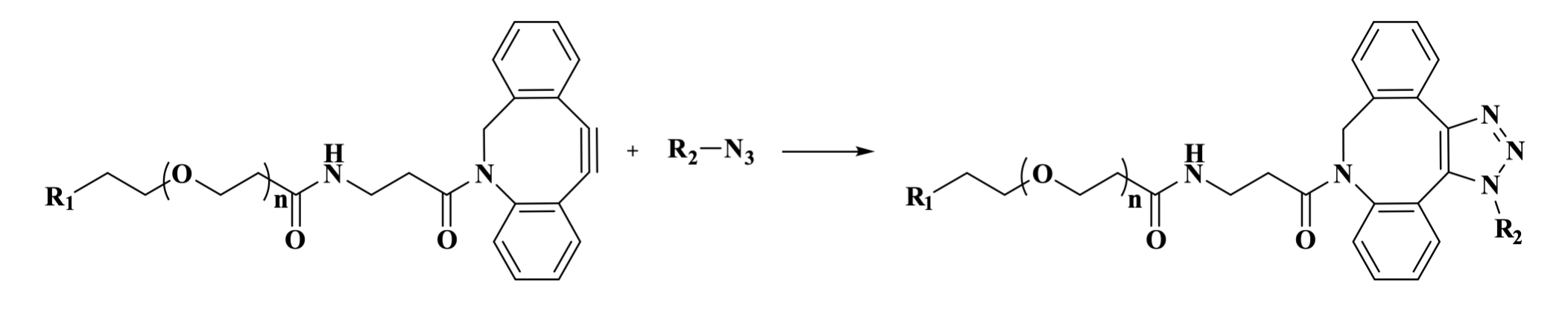
Figure1. The strain-promoted azide-alkyne cycloaddition (SPAAC) between DBCO and an azide.
JSKN003 is a bispecific HER2-targeting antibody-drug conjugate (ADC). It employs DBCO-PEG4-acid (CAS: 1537170-85-6) to covalently conjugate a Gly-Gly-Phe-Gly (CAS: 187794-49-6) tetrapeptide linker to the glycan of a humanized bispecific antibody, and carries a topoisomerase I inhibitor (TOP1i) as the payload. Pre-clinical studies demonstrate strong anti-tumor activity of JSKN003 with superior tolerance and serum stability.
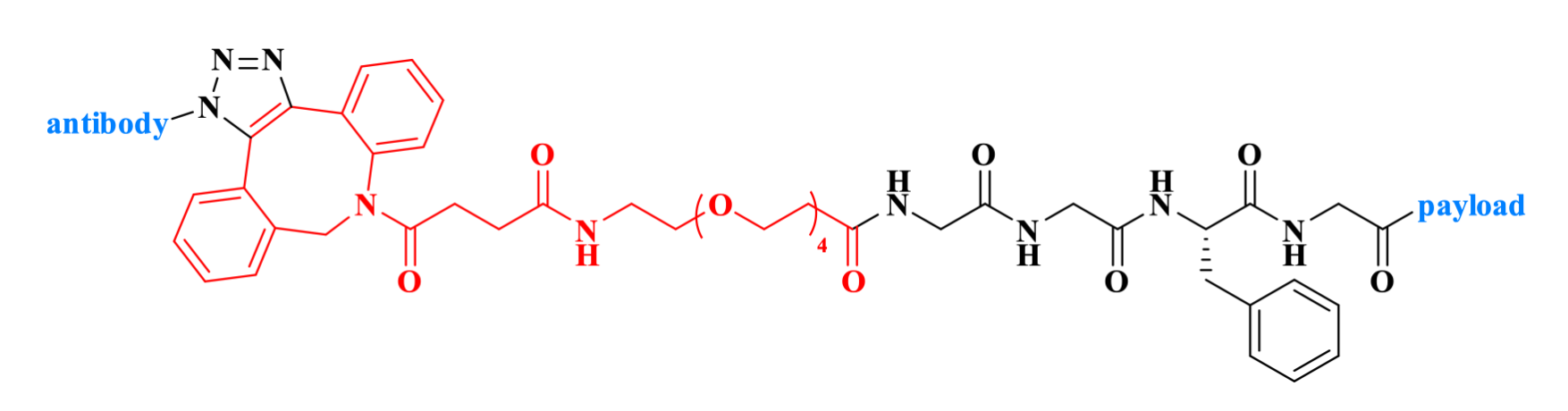
Figure 2. The structure of JSKN003.
Breast tumors are typically composed of distinct cellular subpopulations with varying gene expression profiles. Antibody-drug conjugates (ADCs) represent an emerging class of chemotherapeutic agents with remarkable clinical efficacy; however, tumor heterogeneity and drug resistance in breast cancer pose significant challenges for ADC therapy. By employing branched ADC linkers, payload molecules can be conjugated site-specifically and quantitatively onto a single antibody through orthogonal strain-promoted cycloaddition between DBCO PEG and azides, combined with the Diels-Alder reaction between trans-cyclooctene (TCO) and tetrazine. This dual-drug ADC demonstrates superior in vivo therapeutic efficacy compared to single-warhead ADCs administered alone or in combination.
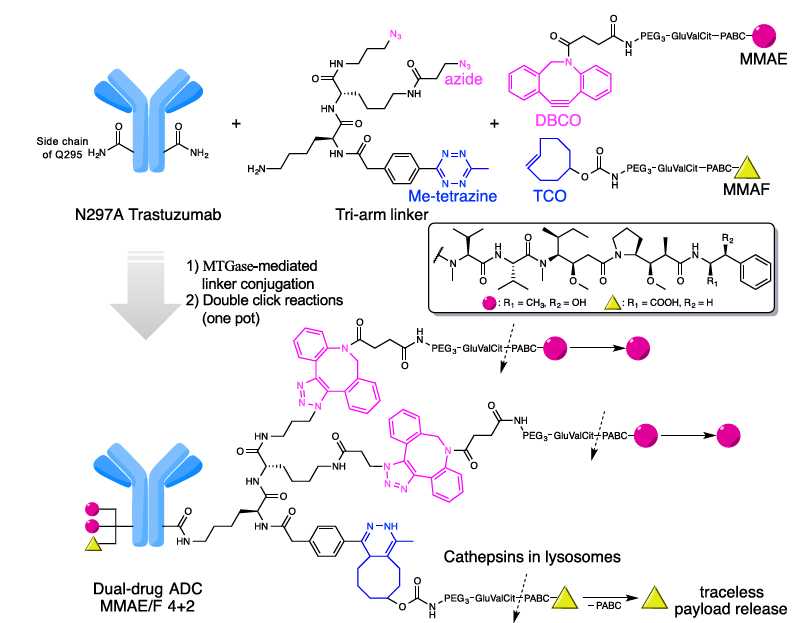
Figure 3. Molecular design and conjugation strategy for generating dual-drug ADCs.
When it comes to design, using DBCO-functionalized PEG linkers in this ADC approach is smart. Strain-promoted azide–alkyne cycloaddition (SPAAC) means you don't need copper catalysts, so you don't have to worry about metal causing harm or breaking down proteins. This is really important for antibody-based treatments, where keeping the structure and activity intact is key.
Plus, the triazole bond that SPAAC creates is super stable in the body, so the drug stays connected to the linker while it's circulating. The PEG spacer also helps with solubility and prevents clumping, which means you can use more of the drug on each antibody without causing safety problems or reducing how well it works. All of this makes DBCO PEG linkers a popular option for creating ADCs that target specific spots and can be used in real-world treatments.
Alkyne PEG is a poly(ethylene glycol) derivative featuring a terminal alkyne group and functional groups, widely used in click chemistry reactions, molecular conjugation, and biomarking. The alkyne group is a highly reactive chemical moiety that can undergo a copper-catalyzed click reaction with azides to form a stable 1,2,3-triazole ring. This reaction is characterized by its high specificity and excellent yield. The PEG chain provides water solubility and biocompatibility while also enhancing molecular stability. Furthermore, the length of the PEG chain can be modulated to meet specific application requirements.
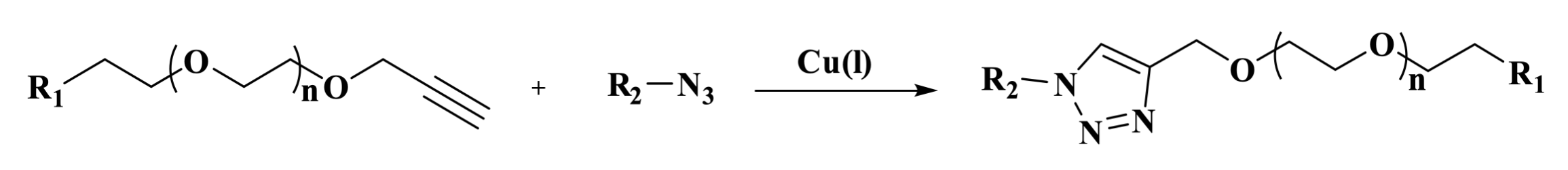
Figure 4. The click reaction between an alkyne and an azide forms a 1,2,3-triazole ring.
Antibody-Drug Conjugates (ADCs) have emerged as a powerful platform for the selective delivery of cytotoxic agents to cancer cells. Using Propargyl-PEG5-acid (CAS: 1245823-51-1), a site-specific ADC with a drug-to-antibody ratio (DAR) of 4 was constructed by conjugating the payload to an anti-EphA2 monoclonal antibody via copper-catalyzed click chemistry. This ADC exhibited potent inhibitory activity against EphA2-positive human prostate cancer cells in both in vitro cytotoxicity assays and a murine tumor xenograft model.
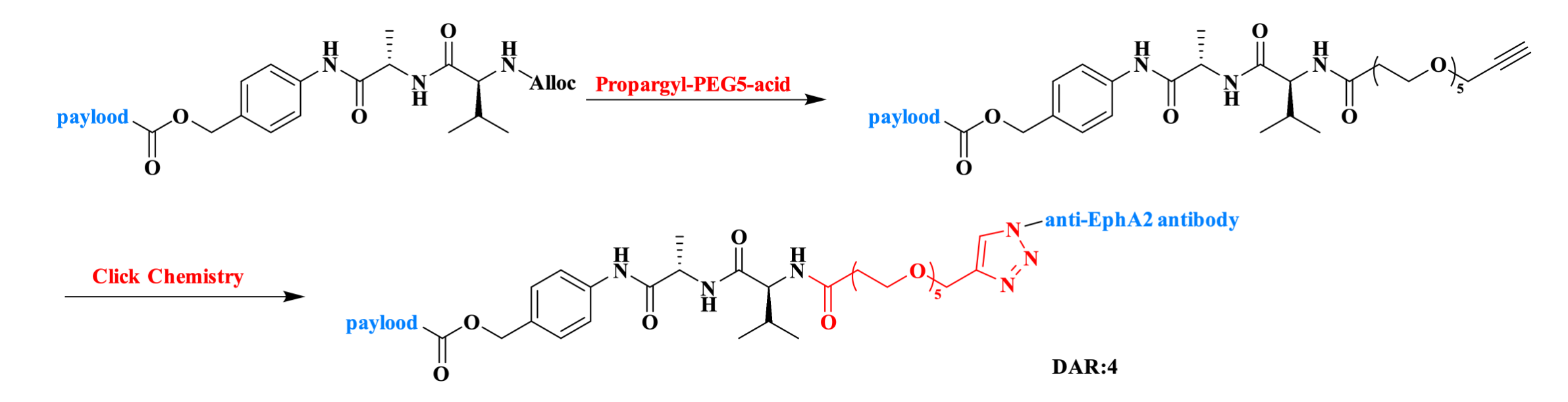
Figure 5. The Synthesis Routes for ADC.
Azide PEG for Click Chemistry
Azide PEG is a highly efficient bifunctional conjugation reagent with unique chemical properties and significant biomedical application value. The azide group exhibits excellent reactivity: it can efficiently couple with alkyne-containing molecules via copper-catalyzed azide-alkyne cycloaddition (CuAAC), and also react rapidly with cycloalkynes such as DBCO or BCN through strain-promoted azide-alkyne cycloaddition (SPAAC), which requires no copper catalyst. Simultaneously, the PEG component confers excellent water solubility and biocompatibility to the azide compound. Azide PEG has been widely adopted in numerous cutting-edge research areas, including the construction of ADCs and PROTACs, the development of targeted drug delivery systems, and the preparation of biomaterials, demonstrating broad application prospects.
Trodelvy is a Trop-2-targeting antibody-drug conjugate (ADC) developed by Gilead Sciences and approved by the U.S. FDA. Trop-2 is a cell surface antigen highly expressed in multiple tumor types, including over 90% of breast and lung cancers. Its linker structure is formed through a cycloaddition reaction between an Azide PEG component and an azide group.
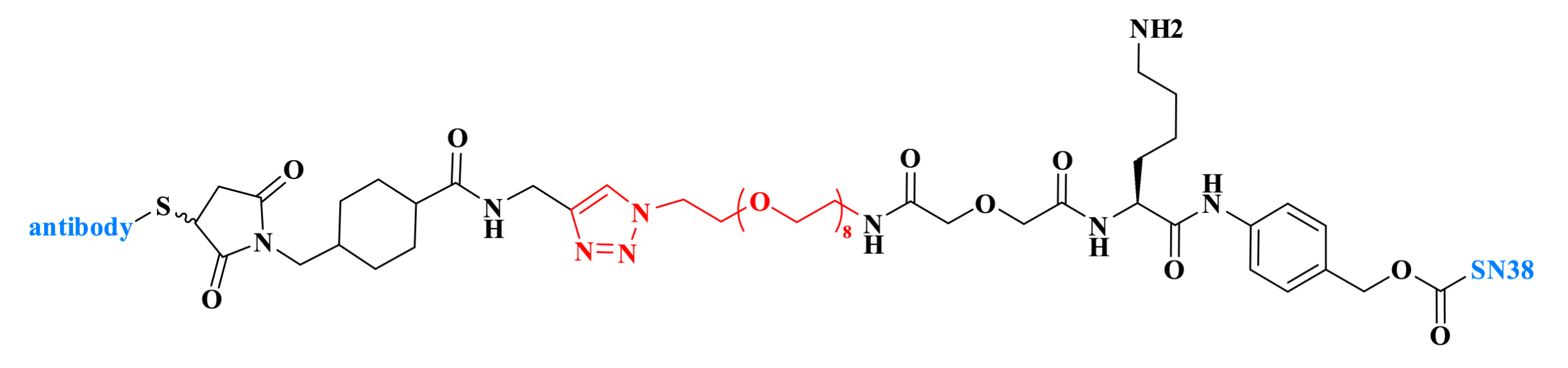
Figure 6. The structure of Trodelvy.
BCN PEG is a class of PEG linkers containing bicyclo[6.1.0]nonyne (BCN), widely used in labeling, bioconjugation, chemical modification, and surface applications. BCN is a highly reactive cycloalkyne that rapidly combines with azide groups via a catalyst-free click reaction (SPAAC) to form a stable triazole ring. The flexible hydrophilic chain, composed of multiple ethylene glycol units, enhances the compound's water solubility and biocompatibility while prolonging its in vivo circulation time. The length of the PEG chain can be customized to modulate water solubility and reaction flexibility.
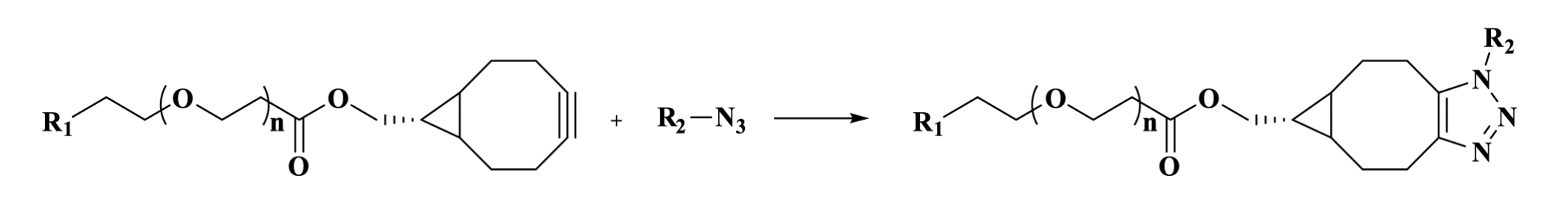
Figure 7. The click reaction between BCN and an azide forms a triazole ring.
Functionalized PEG refers to a class of poly(ethylene glycol) (PEG) derivatives that have been specifically chemically modified, incorporating highly reactive groups such as azide, alkyne, DBCO, or BCN at their terminal ends or side chains. These groups enable them to efficiently participate in click chemistry reactions, particularly copper-catalyzed (CuAAC) or copper-free strain-promoted azide-alkyne cycloaddition (SPAAC). Functionalized PEG has become a key tool in constructing ADCs, developing targeted delivery systems, and modifying biomaterial surfaces, providing a core solution for precise and efficient bioconjugation.
Compared to regular bioconjugation ways, like NHS ester–amine coupling or maleimide–thiol reactions, click chemistry gives you better control over what reacts and how it reacts. Regular methods often have unwanted side reactions, create a mix of different products, and are sensitive to pH or other things that can interfere, especially with complex biomolecules.
Click reactions, on the other hand, usually don't mess with biological stuff and work well in mild, watery conditions. When you combine them with PEG linkers, click chemistry lets you attach things to specific spots while reducing clumping and keeping biological activity intact. That makes PEG-based click chemistry great for things that need to be made consistently and with exact structures, like antibody–drug conjugates, protein labels, and advanced diagnostic tools. Learn more about PEG linker fundamentals and applications to better understand how linker design influences bioconjugation performance.
Yamazaki, Chisato M et al. “Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance.” Nature communications vol. 12,1 3528. 10 Jun. 2021, doi:10.1038/s41467-021-23793-7.
Presolski, Stanislav I et al. “Tailored ligand acceleration of the Cu-catalyzed azide-alkyne cycloaddition reaction: practical and mechanistic implications.” Journal of the American Chemical Society vol. 132,41 (2010): 14570-6. doi:10.1021/ja105743g.
Agard, Nicholas J et al. “A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems.” Journal of the American Chemical Society vol. 126,46 (2004): 15046-7. doi:10.1021/ja044996f.
Thompson, Pamela et al. “Straightforward Glycoengineering Approach to Site-Specific Antibody-Pyrrolobenzodiazepine Conjugates.” ACS medicinal chemistry letters vol. 7,11 1005-1008. 20 Sep. 2016, doi:10.1021/acsmedchemlett.6b00278.
Kolb, Hartmuth C. et al. “Click Chemistry: Diverse Chemical Function from a Few Good Reactions.” Angewandte Chemie (International ed. in English) vol. 40,11 (2001): 2004-2021. doi:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5.