
2025-10-24 Posted by TideChem view:354
A nucleoside is a compound consisting of a five-carbon sugar (e.g., ribose or deoxyribose) linked to a nitrogenous base (e.g., purine or pyrimidine) via a glycosidic bond. A nucleotide is a nucleoside with one or more phosphate groups attached.
Phosphoramidite monomers are key raw materials in the synthesis of small nucleic acid drugs. They primarily combine with different bases (such as adenine, thymine, cytosine, and uracil) to form small nucleic acid molecules. Their synthetic methods allow precise control over the sequence and length of nucleic acid molecules, thereby facilitating the accurate development of small nucleic acid therapeutics. Additionally, the phosphoramidite bond in these monomers exhibits excellent stability, resisting enzymatic degradation in biological systems, which enhances the stability and bioactivity of small nucleic acid drugs.
Small nucleic acid drugs, also known as oligonucleotide therapeutics, are short-chain nucleic acids consisting of ten to several dozen nucleotides that achieve therapeutic effects through gene interference and regulation. Compared to small molecule and antibody drugs, small nucleic acid drugs offer distinct advantages, including shorter development cycles, long-lasting effects, higher success rates in development, and reduced propensity to drug resistance. To date, 22 small nucleic acid drugs have been approved globally, demonstrating significant potential in treating neurological, cardiovascular, and rare diseases.
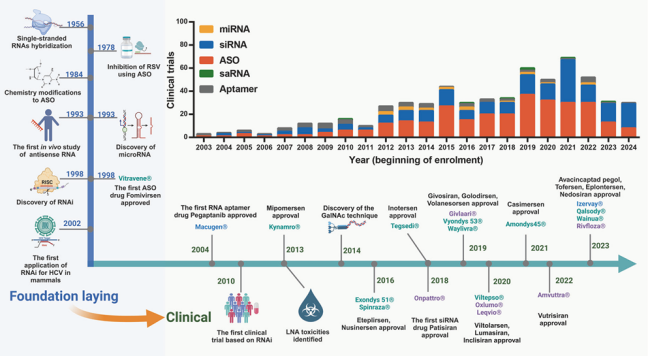
Figure 1. Timeline of milestones from the discovery of small nucleic acid drugs to their clinical use.
Oligonucleotides are synthesized using the solid-supported phosphoramidite method which has been the mainstay of oligonucleotide synthesis for almost 40 years, offering highly efficient and rapid coupling as well as relatively stable starting reagents. The basic starting materials are phosphoramidite monomers derived from protected nucleosides. The synthesis typically proceeds in a 3′ to 5′ direction by a four-step synthesis cycle with each step followed by a solvent wash:
(1) Detritylation: A solution of dichloroacetic acid (DCA) or trichloroacetic acid (TCA) in dichloromethane is used to remove the 5′-DMT protecting group from the terminal nucleotide pre-attached to the solid support, thereby exposing the 5′-OH for subsequent coupling.
(2) Coupling: The next nucleoside phosphoramidite in the sequence is activated with an acidic activator and coupled with the 5′ hydroxyl group of the growing oligonucleotide chain.
(3) Oxidation/Sulfurization: The phosphite triester linkage formed during coupling is susceptible to acid- or base-mediated hydrolysis. Oxygen or sulfur is added to the phosphorus atom using iodine or a sulfurizing reagent to give either a stable phosphodiester or a phosphorothioate group.
(4) Capping: Acetic anhydride and N-methylimidazole are used to form an ester bond with the unreacted 5'-OH groups, prevent further chain extension of coupling failures and minimize N-1 impurities.
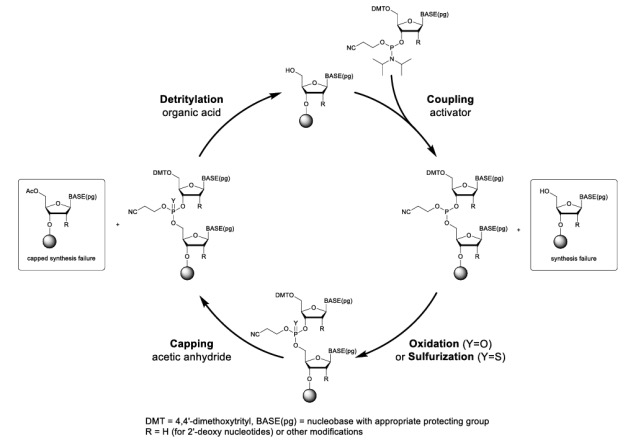
Figure 2. Oligonucleotide synthesis cycle.
Phosphoramidite monomers are the most critical starting materials for small nucleic acid drug synthesis. To increase the binding of small nucleic acid drugs with target sequences, enhance nuclease stability, optimize pharmacokinetic characteristics, and minimize side effects, researchers continue to modify and optimize phosphoramidite monomers.
(1)Backbone modifications
Phosphorothioate (PS) modification, the most prevalent backbone modification, involves substituting a nonbridging oxygen atom in the nucleotide phosphate group with sulfur. While PS modification preserves the pharmacological activity of small nucleic acid drugs, it significantly enhances nuclease resistance, improves plasma protein binding capacity, reduces renal clearance, prolongs in vivo half-life, and optimizes pharmacokinetic properties. In addition, there are two isomers of PS linkage, including right-handed (Rp) and left-handed (Sp), possessing different biological properties.
Likewise, the 3′-bridging oxygen atoms of the PO linkage can be replaced with carbon or nitrogen to form 3′-methylene phosphonate or N3′-phosphoramidate, respectively. In fact, the PO linkage can be completely replaced to form C3-amide, Formacetal、Thioformacetal or MMI.
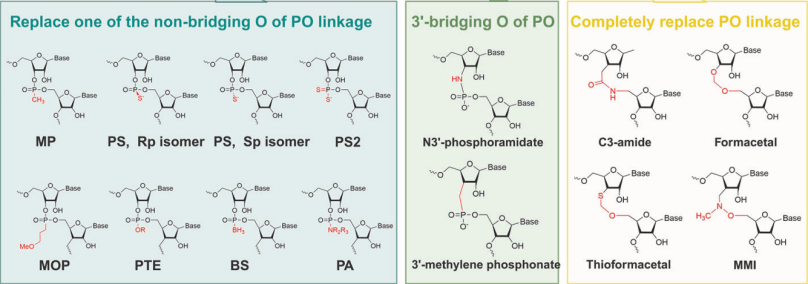
Figure 3. The PO linkage of the backbone can be changed, including the nonbridging O atom, 3’-bridging O atom and total PO linkage.
(2)Ribosome modifications
Ribosome modifications can enhance the binding affinity of small nucleic acid drugs to target RNA, reduce immune response, improve nuclease resistance, and prolong in vivo half-life. Modifications of ribosomes are always performed on the 2’ ends of ribose sugars and sugar chains. Widely used 2’-sugar modifications include 2ʹ-fluoro (2ʹ-F), 2ʹ-O-methyl (2ʹ-OMe) and 2ʹ-O-(2-methoxyethyl) (2ʹ-O-MOE), all of which have been proven to be effective and accessible to the market. Modifications of sugar chain include constrained ethyl (cEt), glycol nucleic acids (GNAs), locked nucleic acids (LNAs) and unlocked nucleic acids (UNAs).
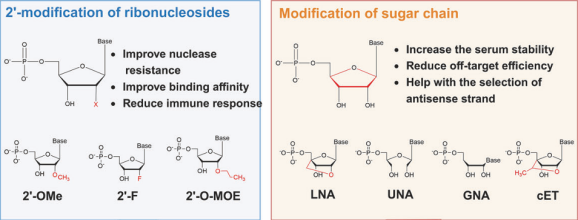
Figure 4. 2’- modification of ribonucleosides and modification of sugar chain.
Novel oligonucleotide analogs, such as tcDNA, PMO, and PNA, significantly enhance both the selectivity and thermal stability of the compounds to increase the binding to target RNAs.
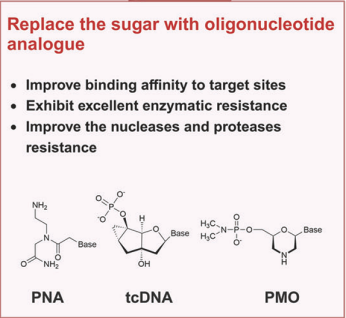
Figure 5. Replace the sugar with oligonucleotide analogue.
(3)Base group modifications
In modifications of the base group, uracil, adenine and cytosine are the common objects. Common modification approaches include methylation, acetylation, Phosphorthioate and hydroxylmethylation, which can significantly influence both the pharmacodynamic and pharmacokinetic properties of small nucleic acid drugs.
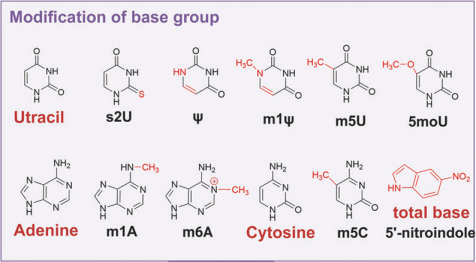
Figure 6. Modification of base group.
Journal Reference
Andrews, Benjamin I et al. “Sustainability Challenges and Opportunities in Oligonucleotide Manufacturing.” The Journal of organic chemistry vol. 86,1 (2021): 49-61. doi:10.1021/acs.joc.0c02291
Liu, Mohan et al. “Landscape of small nucleic acid therapeutics: moving from the bench to the clinic as next-generation medicines.” Signal transduction and targeted therapy vol. 10,1 73. 10 Mar. 2025, doi:10.1038/s41392-024-02112-8.