
2025-11-24 Posted by TideChem view:295
Polysarcosine (Psar) is a water-soluble polymer composed of sarcosine (N-methylglycine) monomers linked via amide bonds. The methyl group present in each repeating unit of its backbone imparts the entire polymer chain with excellent hydrophilicity and electrical neutrality, endowing it with the following outstanding advantages:
(1) Excellent Biocompatibility: Sarcosine is a derivatidve of an endogenous amino acid(Gly) in the human body. Consequently, PSar and its degradation products exhibit very low immunogenicity and biotoxicity.
(2) Half-life Extension Effect: Similar to polyethylene glycol (PEG), PSar can effectively prolong the circulation time of drugs in the body by enhancing drug hydrophilicity and reducing renal filtration.
(3) Biodegradability: Unlike non-degradable PEG, the peptide-like backbone of PSar can be specifically degraded by proteases in vivo, ultimately converting into non-toxic sarcosine that is excreted by the kidneys, thereby avoiding the risk of long-term accumulation.
(4) Precisely Tunable Physicochemical Properties: By controlling the degree of polymerization, the molecular weight and water solubility of PSar can be precisely tailored to meet the requirements of different application scenarios.
Based on these properties, Psar has become a research hotspot in the field of novel biomaterials, demonstrating broad application prospects in drug conjugation, delivery systems, and biomodification, and is hailed as the "next-generation polyethylene glycol."
Eli Lilly's wholly-owned subsidiary, MabLink Bioscience, specializes in the development of antibody-drug conjugates (ADCs). Its core technology platform, PSARLink™, enables the construction of a new generation of ADCs characterized by a homogeneous structure, high plasma stability, and a high drug-to-antibody ratio (DAR), while simultaneously demonstrating excellent pharmacological properties and tolerability in vivo. The cornerstone of this technology is the use of PSar as an innovative hydrophilic linker, which effectively masks the hydrophobicity of the payload, extends the in vivo half-life of the ADC, further optimizes its physicochemical properties, and enhances the inhibitory effect on tumor cells.
Based on the PSARLink™ platform, MabLink has advanced multiple ADC candidates into its development pipeline, with MBK-103 being the most progressed candidate. This investigational drug utilizes a humanized FRα-targeting monoclonal antibody conjugated via the pSar10 (CAS: 2375600-56-7) linker as the hydrophilic spacer, connected through an enzymatically cleavable Val-Ala (CAS: 150114-97-9) dipeptide to the highly potent cytotoxin exatecan. Currently, MBK-103 has demonstrated favorable safety profiles and antitumor activity in clinical trials and has progressed to Phase III clinical studies.
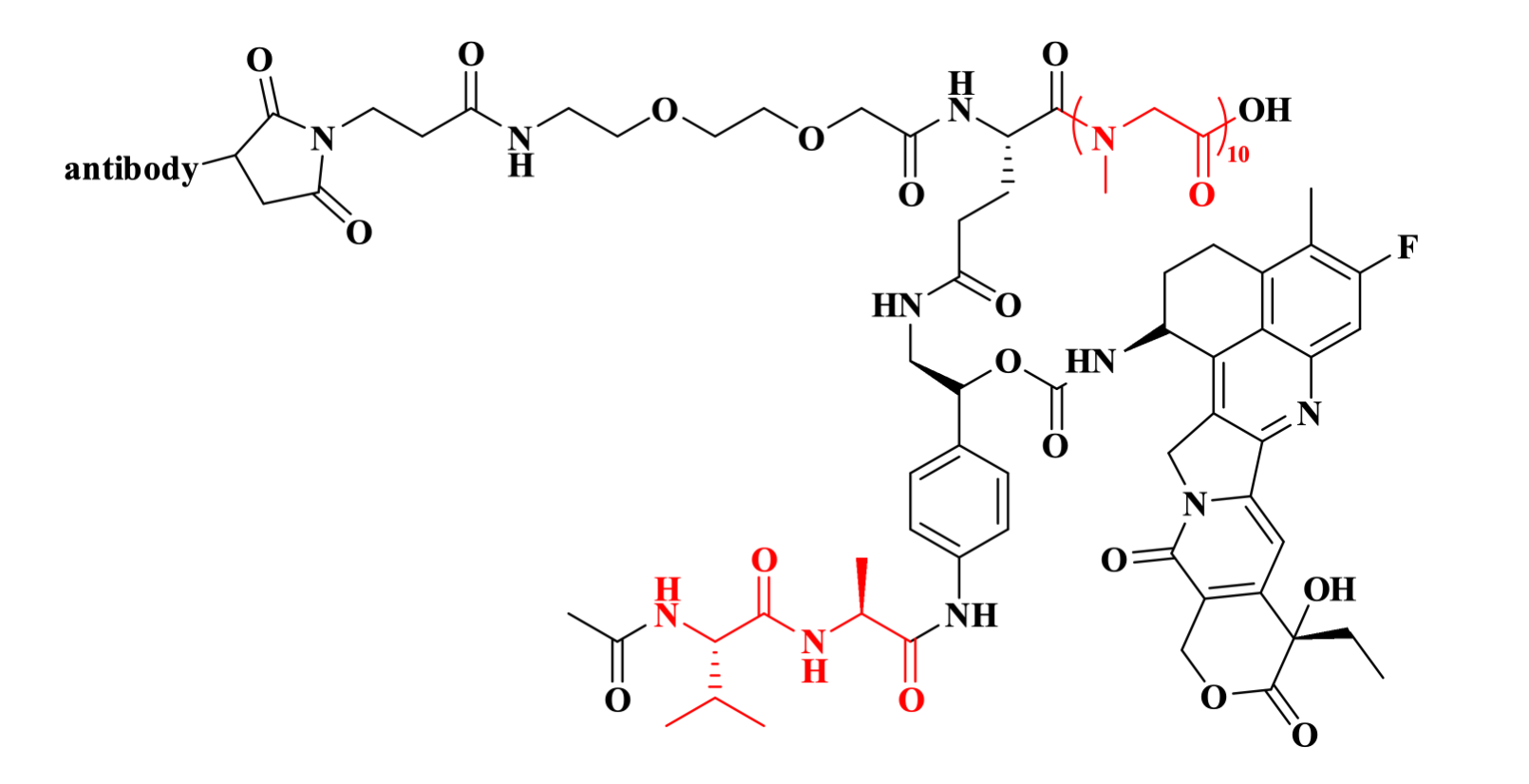
Figure 1. The structure of MBK-103.
BT1718, a Phase I/II clinical candidate drug developed by Bicycle Therapeutics, is a peptide-drug conjugate targeting matrix metalloproteinase-14 (MMP14). It is currently under clinical investigation for indications including non-small cell lung cancer and esophageal cancer. Based on Bicycle's proprietary technology platform, the molecule features a structurally stable bicyclic peptide formed by covalent cross-linking of cysteine residues with organic small-molecule scaffolds such as TBMB, enabling high-affinity and selective binding to MMP14. This targeting peptide is covalently linked via a pSar10 spacer and a reductively cleavable disulfide bond linker to the maytansinoid toxin DM1. Upon cellular internalization into tumor cells, BT1718 undergoes disulfide bond cleavage in the lysosomal acidic environment rich in cathepsin B, leading to the release of the active cytotoxin and exertion of antitumor efficacy.
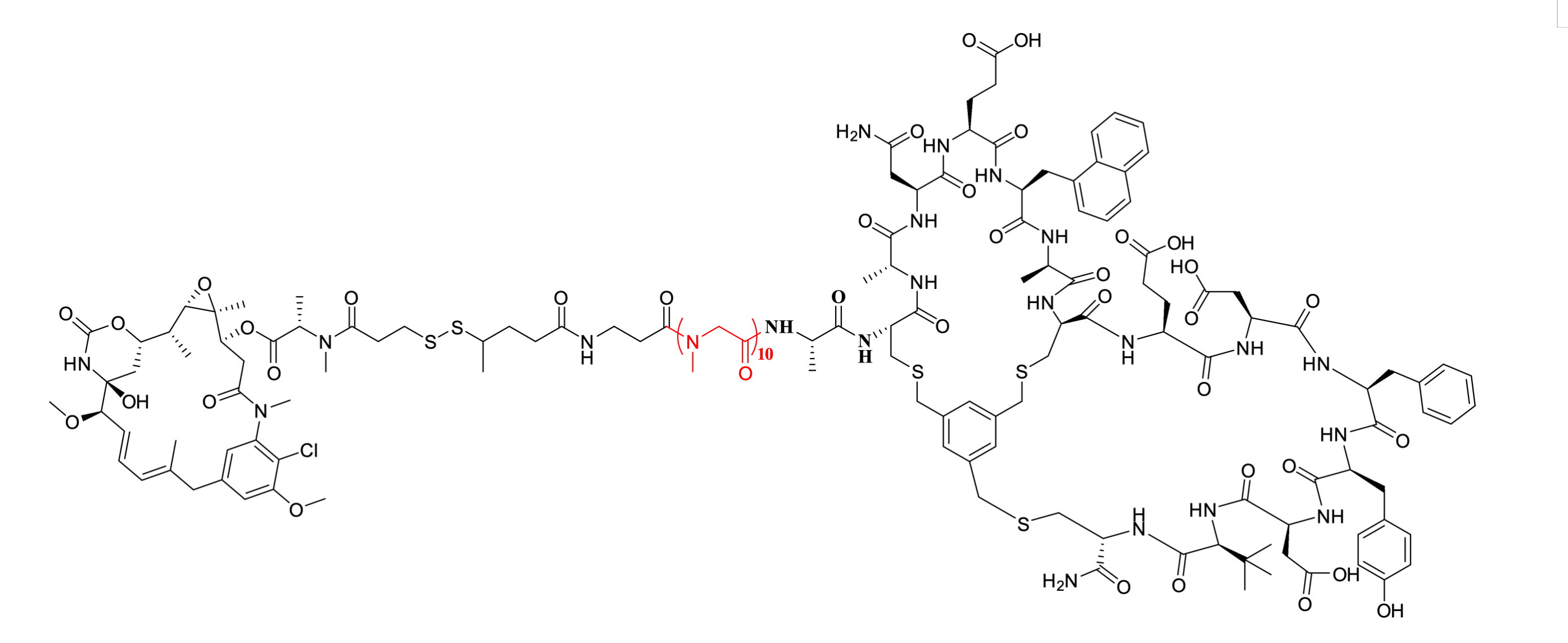
Figure 2. The structure of BT1718.
Current mRNA vaccines widely utilize lipid nanoparticles (LNPs) that typically contain polyethylene glycol (PEG)-lipid components. However, following administration, these vaccines may induce anti-PEG antibodies, which can accelerate blood clearance of LNPs and consequently reduce their delivery efficiency. In severe cases, this may even trigger hypersensitivity reactions. Therefore, developing novel lipid materials capable of replacing PEG-lipids without inducing related immune responses has become an important direction for next-generation mRNA vaccine development.
The research team led by Professor Yizhou Dong has successfully developed novel lipid nanoparticle (LNP) delivery systems using pSar lipids as substitutes for PEG lipids, with SM-102 or ALC-0315 serving as the ionizable lipid components. In mouse models administered with luciferase-encoding mRNA-loaded LNPs via intramuscular injection, pSar-based LNPs (including C16-pSar25, C18-pSar25, TETAMINE-pSar25, and DMG-pSar25) demonstrated comparable or superior in vivo delivery efficiency compared to conventional PEG-based LNPs containing ALC-0159 or DMG-PEG2000. Notably, the expression profiles of 32 different cytokines and chemokines in mice showed similar patterns following injection of LNPs formulated with either pSar or PEG lipids, indicating that pSar lipids do not elicit significant aberrant immunostimulatory characteristics. In conclusion, the pSar-based LNP platform presents considerable potential as a promising PEG-free delivery strategy, offering an important technological pathway for advancing the development of next-generation mRNA therapeutics and warranting further in-depth exploration.
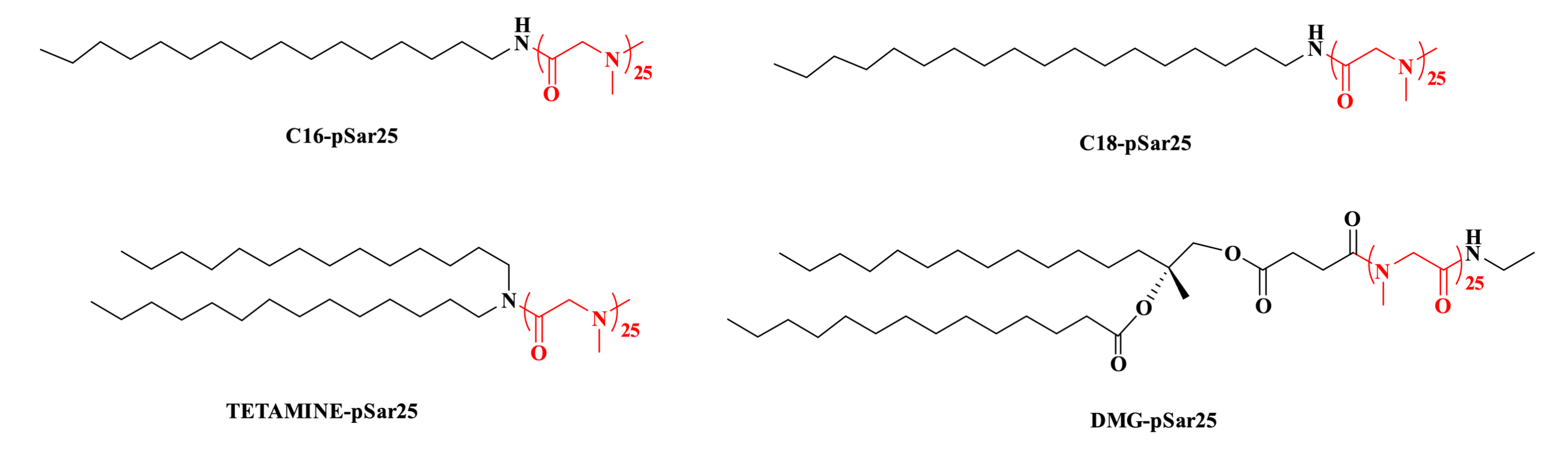
Figure 3. C16-pSar25、C18-pSar25、TETAMINE-pSar25、DMG-pSar25.
Polysarcosine, as an emerging biomaterial, is becoming an ideal delivery platform for next-generation vaccines, cancer therapeutics, and protein drugs due to its excellent biocompatibility, biodegradability, and "stealth" properties. It is regarded as a highly promising alternative to PEG.
Tide Chem consistently supplies high-purity, strictly quality-controlled poly(sarcosine) building blocks, including:
Fmoc-Sar-Sar-OH(CAS:2313534-20-0)
Fmoc-Sar-Sar-Sar-OH(CAS:2749824-37-9)
Fmoc-Sar-Sar-Sar-Sar-OH(CAS:3068545-19-4)
Fmoc-Sar-Sar-Sar-Sar-Sar-Sar-OH(CAS:3061438-88-5)
Fmoc-Sar-Sar-Sar-Sar-Sar-Sar-Sar-Sar-Sar-Sar-OH(CAS:2375600-56-7)
We also offer custom synthesis services to meet specific research needs, providing polysarcosine products with tailored degrees of polymerization and molecular weights to support the development of more precise drug delivery solutions.
Viricel, Warren, et al. "MBK-103, a potent novel conjugation platform-based antibody-drug conjugate, changing therapeutic options in folate receptor alpha positive cancer patients." Cancer Research 83.7_Supplement (2023): 1544-1544.
Harrison, Helen, et al. "BT1718, a novel bicyclic peptide-maytansinoid conjugate targeting MT1-MMP for the treatment of solid tumours: Design of bicyclic peptide and linker selection." Proceedings of the American Association for Cancer Research Annual Conference. 2017.
Bleher, Stefan et al. “Poly(Sarcosine) Surface Modification Imparts Stealth-Like Properties to Liposomes.” Small (Weinheim an der Bergstrasse, Germany) vol. 15,50 (2019): e1904716. doi:10.1002/smll.201904716.
Kang, Diana D et al. “Engineering LNPs with polysarcosine lipids for mRNA delivery.” Bioactive materials vol. 37 86-93. 16 Mar. 2024, doi:10.1016/j.bioactmat.2024.03.017.